Arazy Group Consultants Inc.: Award-winning global registration solutions for the MedTech industry
Produced by Cedrick Adolphe
The future of a medical or diagnostic device manufacturer does not depend only on their ability to innovate, but also on their ability to get their products to market.
This is the principle behind Arazy Group Consultants Inc., an international consultancy spanning several continents that assists medical device companies with mandatory regulatory compliance approvals, especially as they enter new markets.
Founded 20 years ago, Arazy Group operates in over 100 countries worldwide, serving early start-up, medium-sized and top 100 multinational MedTech companies with one mission: to make their medical devices available.
As growth in the medical device sector continues to shift towards emerging markets, and the need to be first to market becomes more important, the regulatory landscape becomes even more complex as companies must dedicate more and more resources to license submissions and maintenance in multiple countries, each with different regulatory frameworks and requirements.
Enter Arazy Group.
“Our disruptive technology is the driving force behind a revolutionary suite of products changing global licensing and registration,” stated Benjamin Arazy, President and CEO. “Since late 2012, we accelerated growth for our clients and made advanced medical devices available to patients and physicians around the globe faster than ever before.”
We recently spoke with Benjamin Arazy to further understand how his company Arazy Group is addressing the growing needs of the MedTech industry in the international market by developing and introducing innovative solutions.
A History of Going Global
Arazy Group was originally established in Israel in 1995 as the first regulatory affairs consulting company in the country. Serving Israel’s medical device industry almost exclusively during the first few years of operation, opportunities for massive growth were soon created.
“Starting in Israel gave us a great advantage,” shared Arazy. “Israel has become a major hub for medical device technology. Working with Israeli manufacturers allowed us to develop unique regulatory knowledge and experience through challenging projects with the new, advanced technology characteristic of the Israeli MedTech sector.”
Working outside of the traditional European Union and American markets also gave Arazy Group the insight they needed to recognize the potential of emerging markets and the role they would play in the future of the MedTech industry. Not only were they among the first to expand their business into developing countries, where there was a greater need for the kind of expertise Arazy Group had to offer, but they were positioned to make an impact right away.
The company started their expansion in China. There, Arazy Group became the first medical device consulting company to obtain European certification (CE Mark) for a Chinese product – a major achievement in 1998. Upon the completion of this landmark project, Arazy Group continued to find similar success in Southeast Asia and Latin America.
After the global economic crisis began in 2008, Arazy Group began to receive inquiries for product registrations from American and European manufacturers who realized that they needed to get their products into more markets to succeed in the new economic environment. It became clear that the skills and system that Arazy Group had built for the developing world were of value to the industry at large and, in response, they set up their international headquarters in Vancouver, British Columbia, Canada.
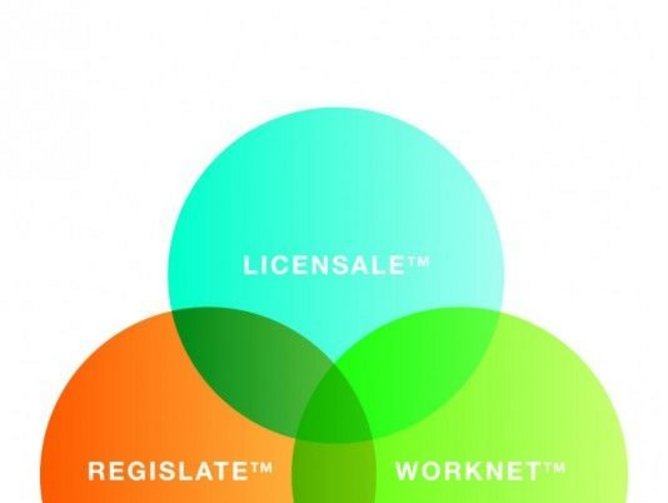
“While we have been operating for two decades within the constraints of the complexity of international regulatory affairs, we have also seen opportunity,” said Arazy. “We combined the power of raw data, the efficiency of data management and the connectivity of a professional social network of compliance experts into a new technology service platform and launched an integrated suite of cloud-based products designed to streamline, simplify and expedite international regulatory affairs once and for all.”
Cloud Computing Excellence
This year, Arazy Group is among the world-class companies honored with the prestigious CIO Impact Award by Frost & Sullivan. Going to companies who enable breakthrough new business models and strategies, Arazy Group was recognized for its cloud-based global MedTech registration system LICENSALE.COM™.
Manufacturers use the LICENSALE.COM™ system to manage their product submissions and submit multiple applications in multiple markets, simultaneously, through one easy-to-use portal. The technology connects Arazy Group’s network of compliance and technical experts to the manufacturers’ registration projects, enabling registration in over 100 countries and reducing time-to-market and overall regulatory costs by as much as 50 percent. The system is designed to address manufacturers’ long-term needs throughout the product lifecycle, including the ongoing maintenance of licenses and registration renewals, and has processed thousands of applications since its official launch in 2012.
“Being recognized by a world-renowned third party for our achievements is extremely important for building our clients’ confidence in our advanced solutions,” said Arazy.
Only weeks before winning the award, Arazy Group introduced the next phase in its suite of integrated cloud-based products: Officially launched at MEDICA 2014 in Düsseldorf, REGISLATE™ is the first dedicated global regulatory management system for the large-scale processing, submission and review of regulated product applications. Directed to manufacturers as well as government agencies or other professional regulatory organizations, REGISLATE™ completes the infrastructure required for the operation of an effective regulatory affairs organization or department.
On February 10, Benjamin Arazy also received the Frost & Sullivan 2015 CIO Innovator of the Year Award. The award goes to those nominees whose teams had the most outstanding impact on their enterprises' strategic innovation, and who delivered a significant competitive advantage.
Constant Innovation
Arazy Group is continuously striving to accelerate time-to-market for faster and equal public access to advanced MedTech products on a global scale.
“Overall, we want to take a leading position with whatever is happening in the field of regulatory affairs, while improving global healthcare through the provision of safe and advanced medical devices and practice” said Arazy.
When asked where he would like to see Arazy Group in the next five years, Benjamin Arazy concluded with the following: “I’d like to see Arazy Group expanding its role as the leading player in the field of regulated product registration to the biotech sphere, followed by other global regulatory verticals.”